8% of children and 2% of adults have food allergy. More than 170 different foods have been reported to cause allergic reactions.
The mechanisms causing food-induced allergic reactions can be:
- IgE mediated
- non-IgE mediated
- related to cellular mechanisms
- associated with eosinophilic inflammation
- a mixture of multiple mechanisms
Management of food allergy is complicated by varied misconceptions within the medical community and the public at large.
Guidelines development for food allergy
Diagnostic tools have primarily focused on IgE-mediated food allergy with implementation of skin prick and allergen-specific IgE testing to make the correct diagnosis, or rule out IgE-mediated disease, as in the case of food protein–induced enterocolitis syndrome (FPIES).
In 2010, the National Institute of Allergy and Infectious Disease published the first “Guidelines on the diagnosis and management of food allergy”.
In 2012, the International Collaboration in Asthma and Allergy assembled an expert panel and published an international consensus. The European Allergy and Asthma guidelines have been published as a working document. A committee formed by the Joint Council of Allergy and Immunology (JCAI) is preparing an updated food allergy practice parameter. The AAAAI has published a guideline about the performance of oral food challenges (OFCs).
New diagnostic tools for food allergy
Component-resolved diagnostics (CRD) testing has recently been approved by the FDA for peanut allergy. CRD uses allergenic proteins derived from recombinant DNA or purification from natural sources to identify sIgE reactivity to recombinant allergenic proteins rather than whole allergen. The current evidence does not support broad clinical use of CRD testing.
sIgE antibodies to Ara h 2 are the most common peanut allergen associated with clinical reactivity. Presence of Ara h 8 alone in patients with birch pollen allergy (with positive Bet v 1 results) might be the source of cross-reactive proteins that increase whole peanut IgE without clinical relevance. However, inconsistencies exist. Different Ara h peanut components have been implicated (in addition to Ara h 2).
CRD did not improve diagnostic accuracy in predicting egg or milk OFC outcomes.
Dietary avoidance and early introduction of food allergens
Many food allergies take long to "outgrow". Even food allergies that are not classically “lifelong” (eg, milk, egg, and soy) are present well into the school-age years for many patients.
Current guidelines for introduction of foods in infants at high risk for food allergy suggest:
- breast-feeding for 4 to 6 months with delayed solid introduction
- use of a hydrolyzed formula for supplementation
- no dietary restrictions in pregnant or lactating women
- delays in allergenic food introduction for children with known food allergy
There is no evidence to make recommendations about the timing of major food allergen introduction.
Food challenges with heated egg and milk proteins in children with milk and egg allergy is an active research area.
New epinephrine autoinjector device
A new epinephrine autoinjector, Auvi-Q (Sanofi) was approved by the FDA n 2012. It has both audio and visual cues that guides the user through each step of the injection process. This device is available in both 0.15- and 0.3-mg doses (same doses as EpiPen).
New billing code for OFC in the U.S. - International Classification of Diseases–ninth (ICD-9) revision coding
The very modest reimbursement for OFC has been a barrier resulting in underuse of this very effective diagnostic tool. Two new codes to better describe ingestion challenge (IG) were approved in 2012 and go into effect January 1, 2013:
- 95076 describes the baseline ingestion (food) challenge and covers the first 2-hour period of an oral challenge; most IGs, particularly open food challenges, fall under this code
- the more complicated IGs last longer than 2 hours, and for these situations, a second add-on code (95079) has been assigned. This code is billed on an hourly basis for the remaining period of the challenge.
For a food challenge that lasts less than 61 minutes total, under CPT rules for timed codes, the clinician cannot bill the IG code but should only bill for this as an evaluation and management service.
JCAAI, representing allergy, has successfully obtained approval for two new codes for ingestion challenge testing - with a significant increase in reimbursement over 2012. The old code – 95075 - will no longer be recognized - effective January 1, 2013. As of that date, the first two hours of an ingestion challenge will be coded 95076 and each additional hour will be coded 95079. The RVUs for 95076 are 3.45, an 85% increase in reimbursement. The RVUs for each hour of additional challenge time beyond two hours and 31 minutes is 2.41 RVUs. Added together, reimbursement for a three-hour challenge will increase approximately 300% over last year. Source: JCAAI (PDF).
Advocacy organizations
Many organizations have worked to improve awareness and raise funds for research. A list of their websites is provided below:
foodallergy.org
aafa.org
kidswithfoodallergies.org
aaaai.org
acaai.org
aaem.org
aap.org
aasa.org
aap.org/sections/schoolhealth
nasn.org
naesp.org
allergyready.com
stlouischildrens.org
schools.allergyhome.org
foodallergybooks.com
allergysupport.org
foodallergyed.com
allergyeats.com
cofarstudy.org
Labeling laws and food safety
The US Food Allergen Labeling and Consumer Protection Act of 2004 (www.fda.gov/Food/FoodSafety/FoodAllergens) requires labeling of foods related to the “major allergens” (egg, milk, wheat, soy, fish, crustacean shellfish, peanut, and tree nuts). A significant labeling exemption is related to highly refined oils derived from food allergens (e.g. peanut oil).
The US law does not regulate the use of advisory labeling, such as “may contain” or “manufactured on equipment with”.
Commercial airline travel safety
The “Top 10 tips for airline travelers with food allergy” are available at foodallergy.org.
Food Safety Modernization Act and Food Allergy and Anaphylaxis Management Act (2011)
This law requires that the US Secretary of Health and Human Services develop and make available to schools a voluntary policy to manage the risk of food allergy and anaphylaxis among students.
School Access to Emergency Epinephrine Act
A few states have laws related to stock epinephrine. 24% of all epinephrine administrations at school were provided to people whose allergy was unknown at the time of administration. Mylan Specialty pharmaceutical company has enacted a program, “EpiPen4Schools,” beginning in August 2012. This program allows for acquisition of up to 4 free EpiPen or EpiPenJr autoinjectors with a prescription, to schools (www.EpiPen4Schools.com).
Research
The terms desensitization and tolerance are often used:
- Desensitization refers to a change in the threshold dose of ingested allergen required to induce allergic symptoms after food exposure occurring during therapy
- Tolerance refers to the long-lasting effects of treatment that persist after the treatment is stopped and allow a patient to fully consume the food
The development of tolerance remains controversial in food allergy therapeutics. The concept of “sustained unresponsiveness” has been introduced. This area is a subject of ongoing research.
Humanized monoclonal anti-IgE
Omalizumab, a recombinant, humanized, anti-IgE mAb therapy has been used in 2 randomized controlled trials for peanut allergy. The trials did not show significant bnefits. Currently, trials are in progress evaluating anti-IgE both as monotherapy and as an adjunct to oral immunotherapy (OIT).
Chinese herbal therapy
Food allergy herbal formula (FAHF-2) contains 9 herbs and is currently in trials. The herbal formula FAHF-2 contains 9 Chinese herbs and is currently in clinical trials for allergy to peanut, tree nuts, sesame, fish, or shellfish.
Oral immunotherapy (OIT)
OIT is associated with clinical and immunologic responses, but it has limitations because of side effects. GI side effects (pain, cramping, nausea, and vomiting) occur in 10-20% of subjects receiving OIT, requiring discontinuation of therapy.
Viral infections, menses, and exercise may decrease the reaction threshold for subjects receiving stable OIT dosing.
Desensitization was associated with:
- increased allergen-specific IgG4 levels
- decreased skin prick test responses
- decreased basophil activation
- decreased TH2 cytokine (IL-5 and IL-13) production
- increased regulatory T-cell numbers with peanut OIT
Specific IgE levels initially increased and then decreased after the first year of study. These changes are similar to the ones observed with the well-established SCIT for airborne allergens.
Tolerance has not been fully defined, or achieved, in clinical trials.
Extensively heated milk and egg protein
A possible alternative or adjunct treatment to OIT is the use of heated allergen to alter protein conformation and reduce IgE binding. Up to 70% of children with milk or egg allergy can safely consume these extensively heated proteins. This is associated with reductions in TH2-type immune responses and accelerated tolerance development.
SLIT
SLIT as a treatment for food allergy has been associated with successes for kiwi, hazelnut, peach, milk, and peanut allergies. Side effects were predominantly oropharyngeal. However, the maximal dose administered in SLIT is limited by the small volume, thus OIT is used in most new studies.
Epicutaneous immunotherapy (EPIT)
EPIT was used in one trial in children with milk allergy. Additional studies are in progress for peanut allergy.
Educational materials
Educational materials related to the 8 most common food allergens are available through foodallergy.org and CoFAR cofargroup.org.
Food allergy programs for schools
“How to C.A.R.E. for students with food allergies: what educators should know” is a free, interactive online course available at AllergyReady.com.
A useful food allergy mnemonic is "CARE":
- Comprehend the basic facts about food allergies
- Avoid the allergen
- Recognize the symptoms of a reaction
- Enact an emergency protocol (Epinephrine)
The Food Allergy Management and Education program for schools was developed by St Louis Children’s Hospital. They have developed a toolkit which is available free of charge at www.stlouischildrens.org.
References:
The changing CARE for patients with food allergy. The Journal of Allergy and Clinical Immunology, Volume 131, Issue 1 , Pages 3-11, January 2013.
http://www.jacionline.org/article/S0091-6749(12)01825-8/fulltext
Showing posts with label Immunotherapy. Show all posts
Showing posts with label Immunotherapy. Show all posts
Immunotherapy (Allergy Shots)
 Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of ChicagoReviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at NSU
Allergy shots (patient information)
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
If you have allergies, you may be wondering if allergy shots are the best treatment for you. Here are the answers to some commonly asked questions.
What exactly is there in the allergy shots?
Allergen extracts are manufactured from natural substances, such as pollens, insect venoms, animal hair, and foods. More than 1,200 extracts are licensed by FDA.
How do allergy shots work?
After allergy testing, typically by skin testing to detect what allergens you or your child may react to, a health care professional injects the child with “extracts” - small amounts of the allergens that trigger a reaction. The doses are gradually increased so that the body builds up immunity to these allergens. Allergy shots work like a vaccine.
There are two phases of administration of allergy shots (immunotherapy): build-up and maintenance.
The build-up phase, ranges from 3 to 6 months, and involves injections with increasing amounts of the allergens. The frequency of injections is once or twice a week. Sometime more rapid build-up schedules are used.
The maintenance phase begins when the most effective dose is reached (maintenance dose). Once the maintenance dose is reached, there are longer periods between injections, typically every 2-4 weeks.
Who administers the allergy shots?
An allergist / immunologist, often referred to as an allergist, is the most qualified physician to test which allergy you have and tell you if allergy shots are right for you. The safest approach is to be seen and treated by a board-certified allergist. You can find an allergist here: http://www.acaai.org/allergist/Pages/locate_an_allergist.aspx
Who needs allergy shots?
Children or adults who don't respond to either over-the-counter (OTC) or prescription medications, or who suffer from frequent complications of allergic rhinitis, may be candidates for allergen immunotherapy - commonly known as allergy shots.
Allergy shots are recommended for patients with allergic asthma, allergic rhinitis/conjunctivitis and stinging insect allergy. They are not recommended for food allergies.
Before a decision is made to begin allergy shots, the following issues must be considered:
- Length of allergy season and the severity of your symptoms
- Whether medications and/or changes to your environment can control your allergy symptoms
- Your desire to avoid long-term medication use
- Time: allergy shots require a major time commitment. The duration is typically 3-5 years, and the shots often require brief clinic visits every 2-4 weeks.
- Cost: may vary depending on your state and insurance coverage
Immunotherapy for children is effective and well tolerated. It prevents the onset of new allergies. Allergy shots are the only treatment that prevents the progression from allergic rhinitis to asthma.
How effective are allergy shots?
According to the National Institute of Allergy and Infectious Diseases (NIAID), about 80-90% of people with allergic rhinitis will see their symptoms and need for medications drop significantly within a year of starting allergy shots.
How quickly will I feel better?
For many people, a decrease in symptoms is seen during the build-up phase, within 3-6 months. For others, it may take as long as 12 months on the maintenance dose.
How long should I stay on allergy shots?
Once the maintenance dose is reached (it takes 3-6 months), allergy shots are continued for 3-5 years. The decision to stop should be discussed with your board-certified allergist. Some people may have a permanent reduction of their allergy symptoms (the best outcome). Others may relapse and then a longer course of allergy shots can be considered.
What are the risks of allergy shots?
There are two types of adverse reactions that can occur with allergy shots.
Local reactions are common and are described as temporary redness and swelling at the injection site. This can happen immediately, or several hours after the treatment.
Systemic reactions are not common, and are usually mild and respond quickly to medications. Signs of a systemic reaction include increased allergy symptoms such as sneezing, stuffy nose or hives. Rarely, a serious systemic reaction called anaphylaxis (an-a-fi-LAK-sis) can develop, with swelling in the throat, wheezing, a feeling of tightness in the chest, nausea or dizziness. This reequires treatment with epinephrine (EpiPen).
Most systemic reactions develop within 30 minutes of giving allergy shots. You should you wait in your allergist office for 30 minutes after your injections.
Is there an alternative to allergy shots such as drops, etc.?
Some doctors are buying extracts licensed for injection and instructing the parents to administer the extracts using a dropper under the adult or child’s tongue. While FDA considers this the practice of medicine (and the agency does not regulate the practice of medicine), parents and patients should be aware that there are no allergenic extracts currently licensed by FDA for oral use.
Can you treat food allergy with allergy shots?
Allergy shots are never appropriate for food allergies. However, it is common to use extracts to test for food allergies so the child or adult can avoid those foods.
References
Allergy Immunotherapy, Allergy Shots. ACAAI.
Allergy Relief for Your Child - FDA Consumer Info.
Allergy Shots: Tips to Remember. AAAAI.
Allergen-specific immunotherapy. Allergy, Asthma & Clinical Immunology 2011, 7(Suppl 1):S5.
Image source: Pollen from a variety of common plants. Dartmouth Electron Microscope Facility, Dartmouth College.
Published: 09/28/2011
Updated: 01/28/2012
Subcutaneous Immunotherapy (SCIT)
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
What is allergen-specific immunotherapy?
The practice of administering to allergic patients gradually increasing doses of an allergen to achieve and maintain hyposensitization toward the allergen. CPT Code 95165 is from the Medicare physician fee schedule for allergy immunotherapy.
Benefits of immunotherapy
- Effective for venom anaphylaxis, allergic rhinconjunctivitis and asthma cause by
inhalant allergen
- Provides long-term benefits - there was still a significant clinical benefit six years
after discontinuation of grass pollen immunotherapy in childhood (Allergy 2002 57:4 306).
- Modifies the natural course of the disease (allergic rhinitis)
- Prevents new sensitizations
- Reduces progression of allergic rhinitis to asthma in children with allergic rhinitis, if started early
Mechanisms of allergen-specific immunotherapy

Mechanisms of allergen-specific immunotherapy (click to enlarge the image). CD27 expression on allergen-specific T cells may be a new surrogate for successful allergen-specific immunotherapy (JACI, 2012).
Cellular mechanisms
- Expands allergen-specific Th1 immunity
- suppresses Th2 response
- Induces regulatory T cells (Tregs) - Tr1 produce IL-10 and Th3 produce TGF-β
- Increases mRNA for Th1 cytokines (IFN-gamma, IL-2, IL-12)
- Reduces mRNA for Th2 cytokines (IL-4)
Successful immunotherapy is often associated with a shift from TH2 to TH1 CD4+ lymphocyte immune response to allergen.
Humoral mechanisms
- Reduction in allergen-specific IgE
- Increase in allergen specific IgG antibodies lead to neutralization of allergen, blockage of IgE-facilitated allergen presentation to T cells, and blockage of IgE-dependent activation of mast cells and basophils
However, increases in allergen-specific IgG blocking antibody titer are not predictive of the duration and degree of efficacy of immunotherapy.
Subcutaneous immunotherapy (SCIT)
Subcutaneous immunotherapy (SCIT) was first introduced by Leonard Noon in 1911. There is a small but real risk of IgE-mediated reactions, including anaphylaxis and death. Systemic reactions occur in 0.05-0.6% of doses administered.
Terms
Other terms that have been used for allergen immunotherapy are hyposensitization, allergen-specific desensitization, and the common terms allergy shots or injections.
Major allergen refers to any antigen that binds to human IgE sera in more than 50% of patients in a clinically sensitive group.
Vaccine, or allergen vaccine, is the recommended term for the therapeutic preparations used in allergen immunotherapy.
Extracts, or allergen extracts, are solutions of proteins and glycoproteins extracted from source materials such as pollen, mold cultures, and pelt.
Desensitization is a process by which effector cells are rendered less reactive or nonreactive to IgE-mediated immune responses by the rapid administration of incremental doses of an allergenic substance.
Rush immunotherapy: incremental doses of allergen are administered at intervals varying between 15 to 30 minutes and 24 hours, until the optimal effective dose is achieved.
Modified rush immunotherapy: subcutaneous allergen injections are administered at 24-hour intervals.
Cluster immunotherapy is the administration of two or more injections per visit to achieve a maintenance dose. It is a type of rush immunotherapy characterized by the giving of several allergen injections in a single day of treatment.
There are Different Build-Up Regimens
1. Conventional / routine (once-twice/week)
2. Daily
3. Cluster (two or more injections per visit)
4. Rush / modified rush / Ultra-rush
Build Up Phase
– Conventional SCIT involves receiving injections with increasing amounts of the allergen. Injections ranges from 1 to 3 times a week.The duration of generally ranges from 3 to 6 months at a frequency of 2 times and 1 time per week, respectively.
– Cluster immunotherapy is an accelerated build-up schedule that entails administering several injections at increasing doses (generally 2-3 per visit) sequentially in a single day of treatment on nonconsecutive days. The maintenance dose is generally achieved within 4 to 8 weeks.
– Rush immunotherapy is an accelerated immunotherapy build-up schedule that entails administering incremental doses of allergen at intervals varying between 15 and 60 minutes over 1 to 3 days until the target therapeutic dose is achieved.
Cluster SCIT
SCIT 1 or 2 times per week with a schedule that contains fewer total injections than are used with conventional immunotherapy.
2 or more injections are given per visit on nonconsecutive days. The injections are typically given at 30-minute intervals. The patient can reach a maintenance dose in 4 weeks.
The cluster schedule is associated with the same or a slightly increased frequency of systemic reactions compared with immunotherapy administered with more conventional schedules.
The occurrence of both local and systemic reactions to cluster immunotherapy can be reduced with administration of an antihistamine 2 hours before dosing.
Rush SCIT
The most accelerated schedule that has been described for inhalant allergens involves
administering 7 injections over the course of 4 hours.
Ultra-rush immunotherapy schedules have been described for stinging insect hypersensitivity to achieve a maintenance dose in as little as 3.5 to 4 hours.
Maintenance Phase for all forms of SCIT (conventional, cluster and rush)
The maintenance phase begins when the effective therapeutic dose is reached. Once the maintenance dose is reached, the intervals between the allergy injections are increased.
The dose generally is the same with each injection, although modifications can be made based on several variables (ie, new vials or a persistent large local reaction causing discomfort).
The intervals between maintenance immunotherapy injections generally ranges from 4 to 8 weeks for venom and every 2 to 4 weeks for inhalant allergens but can be advanced as tolerated if clinical efficacy is maintained.
Definitions for Maintenance Phase SCIT
- Maintenance concentrate - preparation that contains individual or mixtures of manufacturer’s allergen extracts intended for allergen immunotherapy treatment. A maintenance concentrate can be composed of a concentrated dose of a single allergen or a combination of concentrated allergens to prepare an individual patient’s customized allergen immunotherapy extract mixture.
- Maintenance dose (effective therapeutic dose) - the dose that provides therapeutic efficacy without significant adverse local or systemic reactions. The effective therapeutic dose may not be the initially calculated projected effective dose.
- Maintenance goal (projected effective dose) - the allergen dose projected to provide therapeutic efficacy. The maintenance goal is based on published studies, but a projected effective dose has not been established for allergens. Not all patients will tolerate the projected effective dose, and some patients experience therapeutic efficacy at lower doses.
The average duration of immunotherapy is 3-5 years.
Evidence for Efficacy for SCIT (in declining order of efficacy)
- Grass pollens
- Ragweed pollen
- Birch pollen
- Mountain cedar pollen
- Parietaria species pollen
- Cat and dog dander
- Dust mites
Effective Doses of Extracts
Ragweed
4-24 mcg Amb a 1
D. pt
3.25-12 mcg Der p 1
D far
10 mcg Der f 1
Timothy
15-20 mcg Phl p 5
Cat
11-17 mcg Fel d 1
Dog
5 mcg Can f 1
Birch
3.28-12 mcg Bet v 1
Alternaria
1.6 mcg Alt a 1
Standardized extracts are preferred.
- BAU (Bioequivalent Allergy Unit) is based on ID(50) EAL method.
- AU (Allergy Units) was used before BAU and is based upon the major allergen content. Dust mites have AU=BAU.
- Major allergen: Amb a 1, (One FDA unit of Amb a 1 equals 1 mg of Amb a 1, and 350 units of Amb a 1/mL is equivalent to 100,000 BAU/mL). Can f 1 allergen now is also standardized.
Extract concentrations
- WT/Vol - If the extract is 1:10, use 1 ml, if the extract is 1:20, use 2 ml
- Each vial is usually 5 ml or 10 ml
- Dust mite – Use 1 ml of each type & 2ml (max) (5000 AU/ml). If dust mite concentration is 10,000 AU/ml, then use 0.5ml of each type.
- Cat - can use up to 3 ml - up to 30,000 U (10,000 U/ml)
- Bermuda - Use 0.4ml - 4000 U (10,000 U/ml)
- Northern Grasses - Use 0.4 ml - 40,000 U (100,000 U/ml)
- Ragweed - Use 0.5 or 0.6 ml (200 Ag EU/ml)
Venom extract dose
- Vespid venom 100 mcg/ml
- Mixed vespid venom 300 mcg/ml
Commercial honey bee venom vaccine is prepared from venom obtained by electrical stimulation. Commercial vespid venom protein vaccines are prepared by extraction of dissected venom sacs.
Starting Dose in Conventional SCIT
Common starting dilutions from the maintenance concentrate are 1:10,000 (vol/vol) or 1:1000
(vol/vol). Even more diluted concentrations frequently are used for patients who are highly
sensitive, as indicated by history or skin test reaction.
Conventional SCIT Build-up Phase
The frequency of allergen immunotherapy administration during the build-up phase is usually 1 to 2 injections per week.
With this schedule, a typical patient can expect to reach a maintenance dose in 4 to 6 months. The interval between injections is empiric but might be as short as 1 day without any increase in the occurrence of systemic reactions.
Example SCIT schedule:
Vial #4, 1:1000, Schedule A
0.05 ml
0.15 ml
0.25 ml
0.5 ml
Vial #3, 1:100, Schedule B
0.05 ml
0.1 ml
0.2 ml
0.3 ml
0.4 ml
0.5 ml
Vial #2, 1:10, Schedule C
0.05 ml
0.07 ml
0.1 ml
0.2 ml
0.3 ml
0.4 ml
0.5 ml
Vial #1, 1:1, Schedule D
0.05 ml
0.07 ml
0.1 ml
0.15 ml
0.2 ml
0.25 ml
0.3 ml
0.4 ml
0.5 ml
Alternative SCIT schedule
Vial #5, 1:10,000
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #4, 1:1000
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #3, 1:100
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #2, 1:10
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #1, 1:1
0.05, 0.1, 0.2, 0.35, 0.5 (this is maintenance dose)
Dosing adjustments in SCIT
Late or missed doses:
- 7 days or less - no change
- 8-13 days - repeat previous dose
- 14-21 days - decrease dose by 25%
- 21-28 days - decrease dose by 50%
- If the patient missed the injections for more than 6 weeks, go back to the previous vial
New vial - reduce dose by 50%. No need to reduce in venom SCIT.
Systemic reactions - decrease to the last tolerated dose or even lower.
Preservatives of extracts
The glycerinated allergen extract formulation is based on 50% glycerin.
Extracts prepared in saline or buffer solutions with less than 50% glycerin are referred to as aqueous.
Most standardized allergenic extracts are available only as glycerinated products. For most of the standardized products the expiration date is three years from the date of manufacture. The only standardized aqueous product, short ragweed extract, has an expiration dating of 12 months from the date of manufacture.
Non-standardized extracts
- w/v: the weight of allergen source material extracted with a given volume of fluid (weight by
volume)
– 1:100 indicates that 1 g of dry allergen was added to 100 ml of a buffer for extraction
- PNU: protein nitrogen unit, an estimate of the protein nitrogen content of an extract, where 0.01 g of protein nitrogen equals 1 PNU
Extracts with a particular wt/vol or PNU potency can have widely varying biologic activities.
Outdoor molds: Alternaria, Cladosporium, Drechslera (Helminthosporium).
Indoor molds: Penicillium and Aspergillus - they also have the highest extract protease concentration, in the 200s mcg range. Cockroach extract has a protease concentration of 168 mcg. Alternaria is 29 mcg and house dust mite is less than 5 mcg.
Indications for SCIT
- Allergic Rhinitis
- Allergic Asthma
- Venom Allergy
SCIT is Not Indicated
- Atopic Dermatitis
- Food Allergy
Relative Contraindications for SCIT
- Chronic Urticaria/ Angioedema
- Unstable Asthma
- Concurrent use of Beta-blockers (including topicals, e.g. eye drops) or ACE Inhibitors
- Severe Coronary Artery Disease
- Malignancy
- Unable to Communicate Clearly (children younger than 5 years old)
– Significant immunodeficiency
– Severe psychological disorders
– Poor compliance with medications
– Severe obstructive lung disease (limited reserve)
– Conditions that contraindicate epinephrine use
Venom Immunotherapy (VIT)
VIT with 300-mcg doses of mixed vespid venom provides 98% efficacy. Honeybee VIT is 75% to 85% effective.
VIT is generally not necessary in children 16 years of age and younger who have experienced cutaneous systemic reactions without other systemic manifestations.
Adults who have experienced only cutaneous manifestations to an insect sting are generally considered candidates for VIT, although the need for immunotherapy in this group of patients is controversial.
Because the natural history of fire ant hypersensitivity in children who have only cutaneous manifestations has not been well elucidated and there is increased risk of fire ant stings in children who live in areas where fire ants are prevalent, immunotherapy might be considered.
Pregnancy and SCIT
Allergen immunotherapy is usually not initiated during pregnancy because of risks associated with systemic reactions and their treatment (ie, spontaneous abortion, premature labor, or fetal hypoxia).
The initiation of immunotherapy might be considered during pregnancy for a high-risk medical condition, such as anaphylaxis caused by Hymenoptera hypersensitivity.
When a patient receiving immunotherapy reports that she is pregnant, the dose of immunotherapy is usually not increased, and the patient is maintained on the dose that she is receiving at that time.
Allergists should provide an EpiPen prescriptions to all patients on SCIT.
References
Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. JACI, 2010.
Related reading
Immunotherapy reduces asthma symptoms and use of asthma medication. Cochrane Review, 2010. http://bit.ly/bXXITA - http://bit.ly/bRNiXY - http://bit.ly/bED4BH
Allergen Immunotherapy: A History of the First 100 Years. Medscape, 2011.
Immunotherapy can provide lasting relief - AAAAI info sheet for patients (PDF).
Dose adjustments for patients late for allergen immunotherapy injections. AAAAI Ask The Expert, 2010. http://goo.gl/je9tE
Pollen immunotherapy induces allergen-specific IgG antibodies with inhibitory activity against IgE that are persistent. JACI, 2011.
Your allergy meds may be making you fat - Regular use of OTC antihistamines has been linked to weight gain. NBC, 2011.
SCIT ("allergy shots") is at least as potent as pharmacotherapy in controlling the symptoms of allergic rhintis as early as the first season of therapy. JACI, 2011.
100 years since Leonard Noon published historic paper on SCIT “PROPHYLACTIC INOCULATION AGAINST HAY FEVER” in the Lancet http://goo.gl/Zw3FG
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Best Immunotherapy for Allergic Rhinitis and Asthma: SCIT or SLIT? SCIT is likely more effective, SLIT is safer. Medscape, 2011.
Allergen-specific immunotherapy. Allergy, Asthma & Clinical Immunology 2011, 7(Suppl 1):S5.
Allergy immunotherapy: Reduced health care costs in adults and children with allergic rhinitis. http://buff.ly/12sDYdh
Published: 06/27/2010
Updated: 02/27/2013
Reviewer: S. Randhawa, M.D., Allergist/Immunologist
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
What is allergen-specific immunotherapy?
The practice of administering to allergic patients gradually increasing doses of an allergen to achieve and maintain hyposensitization toward the allergen. CPT Code 95165 is from the Medicare physician fee schedule for allergy immunotherapy.
Benefits of immunotherapy
- Effective for venom anaphylaxis, allergic rhinconjunctivitis and asthma cause by
inhalant allergen
- Provides long-term benefits - there was still a significant clinical benefit six years
after discontinuation of grass pollen immunotherapy in childhood (Allergy 2002 57:4 306).
- Modifies the natural course of the disease (allergic rhinitis)
- Prevents new sensitizations
- Reduces progression of allergic rhinitis to asthma in children with allergic rhinitis, if started early
Mechanisms of allergen-specific immunotherapy
Mechanisms of allergen-specific immunotherapy (click to enlarge the image). CD27 expression on allergen-specific T cells may be a new surrogate for successful allergen-specific immunotherapy (JACI, 2012).
Cellular mechanisms
- Expands allergen-specific Th1 immunity
- suppresses Th2 response
- Induces regulatory T cells (Tregs) - Tr1 produce IL-10 and Th3 produce TGF-β
- Increases mRNA for Th1 cytokines (IFN-gamma, IL-2, IL-12)
- Reduces mRNA for Th2 cytokines (IL-4)
Successful immunotherapy is often associated with a shift from TH2 to TH1 CD4+ lymphocyte immune response to allergen.
Humoral mechanisms
- Reduction in allergen-specific IgE
- Increase in allergen specific IgG antibodies lead to neutralization of allergen, blockage of IgE-facilitated allergen presentation to T cells, and blockage of IgE-dependent activation of mast cells and basophils
However, increases in allergen-specific IgG blocking antibody titer are not predictive of the duration and degree of efficacy of immunotherapy.
Subcutaneous immunotherapy (SCIT)
Subcutaneous immunotherapy (SCIT) was first introduced by Leonard Noon in 1911. There is a small but real risk of IgE-mediated reactions, including anaphylaxis and death. Systemic reactions occur in 0.05-0.6% of doses administered.
Terms
Other terms that have been used for allergen immunotherapy are hyposensitization, allergen-specific desensitization, and the common terms allergy shots or injections.
Major allergen refers to any antigen that binds to human IgE sera in more than 50% of patients in a clinically sensitive group.
Vaccine, or allergen vaccine, is the recommended term for the therapeutic preparations used in allergen immunotherapy.
Extracts, or allergen extracts, are solutions of proteins and glycoproteins extracted from source materials such as pollen, mold cultures, and pelt.
Desensitization is a process by which effector cells are rendered less reactive or nonreactive to IgE-mediated immune responses by the rapid administration of incremental doses of an allergenic substance.
Rush immunotherapy: incremental doses of allergen are administered at intervals varying between 15 to 30 minutes and 24 hours, until the optimal effective dose is achieved.
Modified rush immunotherapy: subcutaneous allergen injections are administered at 24-hour intervals.
Cluster immunotherapy is the administration of two or more injections per visit to achieve a maintenance dose. It is a type of rush immunotherapy characterized by the giving of several allergen injections in a single day of treatment.
There are Different Build-Up Regimens
1. Conventional / routine (once-twice/week)
2. Daily
3. Cluster (two or more injections per visit)
4. Rush / modified rush / Ultra-rush
Build Up Phase
– Conventional SCIT involves receiving injections with increasing amounts of the allergen. Injections ranges from 1 to 3 times a week.The duration of generally ranges from 3 to 6 months at a frequency of 2 times and 1 time per week, respectively.
– Cluster immunotherapy is an accelerated build-up schedule that entails administering several injections at increasing doses (generally 2-3 per visit) sequentially in a single day of treatment on nonconsecutive days. The maintenance dose is generally achieved within 4 to 8 weeks.
– Rush immunotherapy is an accelerated immunotherapy build-up schedule that entails administering incremental doses of allergen at intervals varying between 15 and 60 minutes over 1 to 3 days until the target therapeutic dose is achieved.
Cluster SCIT
SCIT 1 or 2 times per week with a schedule that contains fewer total injections than are used with conventional immunotherapy.
2 or more injections are given per visit on nonconsecutive days. The injections are typically given at 30-minute intervals. The patient can reach a maintenance dose in 4 weeks.
The cluster schedule is associated with the same or a slightly increased frequency of systemic reactions compared with immunotherapy administered with more conventional schedules.
The occurrence of both local and systemic reactions to cluster immunotherapy can be reduced with administration of an antihistamine 2 hours before dosing.
Rush SCIT
The most accelerated schedule that has been described for inhalant allergens involves
administering 7 injections over the course of 4 hours.
Ultra-rush immunotherapy schedules have been described for stinging insect hypersensitivity to achieve a maintenance dose in as little as 3.5 to 4 hours.
Maintenance Phase for all forms of SCIT (conventional, cluster and rush)
The maintenance phase begins when the effective therapeutic dose is reached. Once the maintenance dose is reached, the intervals between the allergy injections are increased.
The dose generally is the same with each injection, although modifications can be made based on several variables (ie, new vials or a persistent large local reaction causing discomfort).
The intervals between maintenance immunotherapy injections generally ranges from 4 to 8 weeks for venom and every 2 to 4 weeks for inhalant allergens but can be advanced as tolerated if clinical efficacy is maintained.
Definitions for Maintenance Phase SCIT
- Maintenance concentrate - preparation that contains individual or mixtures of manufacturer’s allergen extracts intended for allergen immunotherapy treatment. A maintenance concentrate can be composed of a concentrated dose of a single allergen or a combination of concentrated allergens to prepare an individual patient’s customized allergen immunotherapy extract mixture.
- Maintenance dose (effective therapeutic dose) - the dose that provides therapeutic efficacy without significant adverse local or systemic reactions. The effective therapeutic dose may not be the initially calculated projected effective dose.
- Maintenance goal (projected effective dose) - the allergen dose projected to provide therapeutic efficacy. The maintenance goal is based on published studies, but a projected effective dose has not been established for allergens. Not all patients will tolerate the projected effective dose, and some patients experience therapeutic efficacy at lower doses.
The average duration of immunotherapy is 3-5 years.
Evidence for Efficacy for SCIT (in declining order of efficacy)
- Grass pollens
- Ragweed pollen
- Birch pollen
- Mountain cedar pollen
- Parietaria species pollen
- Cat and dog dander
- Dust mites
Effective Doses of Extracts
Ragweed
4-24 mcg Amb a 1
D. pt
3.25-12 mcg Der p 1
D far
10 mcg Der f 1
Timothy
15-20 mcg Phl p 5
Cat
11-17 mcg Fel d 1
Dog
5 mcg Can f 1
Birch
3.28-12 mcg Bet v 1
Alternaria
1.6 mcg Alt a 1
Standardized extracts are preferred.
- BAU (Bioequivalent Allergy Unit) is based on ID(50) EAL method.
- AU (Allergy Units) was used before BAU and is based upon the major allergen content. Dust mites have AU=BAU.
- Major allergen: Amb a 1, (One FDA unit of Amb a 1 equals 1 mg of Amb a 1, and 350 units of Amb a 1/mL is equivalent to 100,000 BAU/mL). Can f 1 allergen now is also standardized.
Extract concentrations
- WT/Vol - If the extract is 1:10, use 1 ml, if the extract is 1:20, use 2 ml
- Each vial is usually 5 ml or 10 ml
- Dust mite – Use 1 ml of each type & 2ml (max) (5000 AU/ml). If dust mite concentration is 10,000 AU/ml, then use 0.5ml of each type.
- Cat - can use up to 3 ml - up to 30,000 U (10,000 U/ml)
- Bermuda - Use 0.4ml - 4000 U (10,000 U/ml)
- Northern Grasses - Use 0.4 ml - 40,000 U (100,000 U/ml)
- Ragweed - Use 0.5 or 0.6 ml (200 Ag EU/ml)
Venom extract dose
- Vespid venom 100 mcg/ml
- Mixed vespid venom 300 mcg/ml
Commercial honey bee venom vaccine is prepared from venom obtained by electrical stimulation. Commercial vespid venom protein vaccines are prepared by extraction of dissected venom sacs.
Starting Dose in Conventional SCIT
Common starting dilutions from the maintenance concentrate are 1:10,000 (vol/vol) or 1:1000
(vol/vol). Even more diluted concentrations frequently are used for patients who are highly
sensitive, as indicated by history or skin test reaction.
Conventional SCIT Build-up Phase
The frequency of allergen immunotherapy administration during the build-up phase is usually 1 to 2 injections per week.
With this schedule, a typical patient can expect to reach a maintenance dose in 4 to 6 months. The interval between injections is empiric but might be as short as 1 day without any increase in the occurrence of systemic reactions.
Example SCIT schedule:
Vial #4, 1:1000, Schedule A
0.05 ml
0.15 ml
0.25 ml
0.5 ml
Vial #3, 1:100, Schedule B
0.05 ml
0.1 ml
0.2 ml
0.3 ml
0.4 ml
0.5 ml
Vial #2, 1:10, Schedule C
0.05 ml
0.07 ml
0.1 ml
0.2 ml
0.3 ml
0.4 ml
0.5 ml
Vial #1, 1:1, Schedule D
0.05 ml
0.07 ml
0.1 ml
0.15 ml
0.2 ml
0.25 ml
0.3 ml
0.4 ml
0.5 ml
Alternative SCIT schedule
Vial #5, 1:10,000
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #4, 1:1000
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #3, 1:100
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #2, 1:10
0.05, 0.1, 0.2, 0.35, 0.5 ml
Vial #1, 1:1
0.05, 0.1, 0.2, 0.35, 0.5 (this is maintenance dose)
Dosing adjustments in SCIT
Late or missed doses:
- 7 days or less - no change
- 8-13 days - repeat previous dose
- 14-21 days - decrease dose by 25%
- 21-28 days - decrease dose by 50%
- If the patient missed the injections for more than 6 weeks, go back to the previous vial
New vial - reduce dose by 50%. No need to reduce in venom SCIT.
Systemic reactions - decrease to the last tolerated dose or even lower.
Preservatives of extracts
The glycerinated allergen extract formulation is based on 50% glycerin.
Extracts prepared in saline or buffer solutions with less than 50% glycerin are referred to as aqueous.
Most standardized allergenic extracts are available only as glycerinated products. For most of the standardized products the expiration date is three years from the date of manufacture. The only standardized aqueous product, short ragweed extract, has an expiration dating of 12 months from the date of manufacture.
Non-standardized extracts
- w/v: the weight of allergen source material extracted with a given volume of fluid (weight by
volume)
– 1:100 indicates that 1 g of dry allergen was added to 100 ml of a buffer for extraction
- PNU: protein nitrogen unit, an estimate of the protein nitrogen content of an extract, where 0.01 g of protein nitrogen equals 1 PNU
Extracts with a particular wt/vol or PNU potency can have widely varying biologic activities.
Outdoor molds: Alternaria, Cladosporium, Drechslera (Helminthosporium).
Indoor molds: Penicillium and Aspergillus - they also have the highest extract protease concentration, in the 200s mcg range. Cockroach extract has a protease concentration of 168 mcg. Alternaria is 29 mcg and house dust mite is less than 5 mcg.
Indications for SCIT
- Allergic Rhinitis
- Allergic Asthma
- Venom Allergy
SCIT is Not Indicated
- Atopic Dermatitis
- Food Allergy
Relative Contraindications for SCIT
- Chronic Urticaria/ Angioedema
- Unstable Asthma
- Concurrent use of Beta-blockers (including topicals, e.g. eye drops) or ACE Inhibitors
- Severe Coronary Artery Disease
- Malignancy
- Unable to Communicate Clearly (children younger than 5 years old)
– Significant immunodeficiency
– Severe psychological disorders
– Poor compliance with medications
– Severe obstructive lung disease (limited reserve)
– Conditions that contraindicate epinephrine use
Venom Immunotherapy (VIT)
VIT with 300-mcg doses of mixed vespid venom provides 98% efficacy. Honeybee VIT is 75% to 85% effective.
VIT is generally not necessary in children 16 years of age and younger who have experienced cutaneous systemic reactions without other systemic manifestations.
Adults who have experienced only cutaneous manifestations to an insect sting are generally considered candidates for VIT, although the need for immunotherapy in this group of patients is controversial.
Because the natural history of fire ant hypersensitivity in children who have only cutaneous manifestations has not been well elucidated and there is increased risk of fire ant stings in children who live in areas where fire ants are prevalent, immunotherapy might be considered.
Pregnancy and SCIT
Allergen immunotherapy is usually not initiated during pregnancy because of risks associated with systemic reactions and their treatment (ie, spontaneous abortion, premature labor, or fetal hypoxia).
The initiation of immunotherapy might be considered during pregnancy for a high-risk medical condition, such as anaphylaxis caused by Hymenoptera hypersensitivity.
When a patient receiving immunotherapy reports that she is pregnant, the dose of immunotherapy is usually not increased, and the patient is maintained on the dose that she is receiving at that time.
Allergists should provide an EpiPen prescriptions to all patients on SCIT.
References
Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. JACI, 2010.
Related reading
Immunotherapy reduces asthma symptoms and use of asthma medication. Cochrane Review, 2010. http://bit.ly/bXXITA - http://bit.ly/bRNiXY - http://bit.ly/bED4BH
Allergen Immunotherapy: A History of the First 100 Years. Medscape, 2011.
Immunotherapy can provide lasting relief - AAAAI info sheet for patients (PDF).
Dose adjustments for patients late for allergen immunotherapy injections. AAAAI Ask The Expert, 2010. http://goo.gl/je9tE
Pollen immunotherapy induces allergen-specific IgG antibodies with inhibitory activity against IgE that are persistent. JACI, 2011.
Your allergy meds may be making you fat - Regular use of OTC antihistamines has been linked to weight gain. NBC, 2011.
SCIT ("allergy shots") is at least as potent as pharmacotherapy in controlling the symptoms of allergic rhintis as early as the first season of therapy. JACI, 2011.
100 years since Leonard Noon published historic paper on SCIT “PROPHYLACTIC INOCULATION AGAINST HAY FEVER” in the Lancet http://goo.gl/Zw3FG
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Best Immunotherapy for Allergic Rhinitis and Asthma: SCIT or SLIT? SCIT is likely more effective, SLIT is safer. Medscape, 2011.
Allergen-specific immunotherapy. Allergy, Asthma & Clinical Immunology 2011, 7(Suppl 1):S5.
Allergy immunotherapy: Reduced health care costs in adults and children with allergic rhinitis. http://buff.ly/12sDYdh
Published: 06/27/2010
Updated: 02/27/2013
New approaches to immunotherapy
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at NSU
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).

Mechanisms of allergen-specific immunotherapy (click to enlarge the image).
Novel approaches to immunotherapy
- Sublingual immunotherapy (SLIT)
- Peptide-based immunotherapy
- CpG-enhanced immunotherapy
- Anti-IgE and immunotherapy
- Recombinant allergen vaccines
- Epicutaneous allergen-specific immunotherapy. Skin patches coated with allergens to treat hay fever - check the caveats too listed by WSJ and JACI, 2011.
SLIT
Interesting fact: The longest human tongue ever recorded was that of Stephen Taylor and measures 9.8 centimetres (3.86 in). The longest tongue for a female is that of Annika Irmler at 7 centimetres (2.76 in).
The World Health Organization concluded that SLIT was a viable alternative to SCIT in 1998.
Practical aspects of SLIT
- Self-administered by patients
- Soluble tablets or drops
- Sublingual-swallow: keep under the tongue for 1-2 min, then swallow. Contact time with the oral mucosa is critical to the effectiveness of SLIT - if the allergen is immediately swallowed, there are negligible clinical effects.
- Dose of allergen greater than for SCIT (3-300 times higher)
- Administration schedule and amount of allergen vary, depending on the manufacturer
- Amount of allergen given during a course of SLIT is higher than in SCIT: there is a bild-up phase (very rapid), followed by a maintenance phase
Efficacy of SLIT in allergic rhinitis
The magnitude of clinical efficacy ranged from 20-60% reduction of symptoms. House dust mite (HDM) SLIT was less effective, but results were comparable to SCIT. For HDM, duration of treatment seems to be crucial - treatments that lasted longer than 24 months had positive results.
Clinical safety of SCIT vs. SLIT
Safety of SCIT
Systemic reactions occur in 0.05-0.6% of doses administered. Rare fatalities have been reported.
Safety of SLIT
No life-threatening adverse events reported since 1986. Most common adverse effects include oral/sublingual itching, stomachache, and nausea. No increased risk in patients with oral allergy syndrome.
Sublingual immunotherapy is safe during pregnancy, it is also safe when initiated for the first time in pregnancy (study) (Allergy, 2012).
CpG-enhanced immunotherapy
Bacterial DNA contains unmethylated CpG di-nucleotides (CpG motifs) that act as a danger signal to the vertebrate immune system and trigger protective innate and acquired immune responses.
CpGs work through toll-like receptor 9 (TLR-9) to activate monocytes, dendritic cells, B cells, and NK cells. CPGs promote Th1 and inhibit Th2 response (similar to allergen-specific SCIT). TLR9 and MyD88 play central role in protective immunity to malaria http://goo.gl/VVF07
The immune stimulatory activity of bacterial DNA can be mimicked by synthetic oligodeoxynucleotides containing CpG motifs. They can be added to a vaccine or linked to an
antigen to greatly boost the immune response.
Anti-IgE (omalizumab (Xoliar) and immunotherapy
The combination anti-IgE and allergen immunotherapy might offer advantages that
neither method can provide separately. Anti-IgE administered during the induction phase
of immunotherapy might reduce the risk of IgE-mediated anaphylaxis.
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at NSU
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
Mechanisms of allergen-specific immunotherapy (click to enlarge the image).
Novel approaches to immunotherapy
- Sublingual immunotherapy (SLIT)
- Peptide-based immunotherapy
- CpG-enhanced immunotherapy
- Anti-IgE and immunotherapy
- Recombinant allergen vaccines
- Epicutaneous allergen-specific immunotherapy. Skin patches coated with allergens to treat hay fever - check the caveats too listed by WSJ and JACI, 2011.
SLIT
Interesting fact: The longest human tongue ever recorded was that of Stephen Taylor and measures 9.8 centimetres (3.86 in). The longest tongue for a female is that of Annika Irmler at 7 centimetres (2.76 in).
The World Health Organization concluded that SLIT was a viable alternative to SCIT in 1998.
Practical aspects of SLIT
- Self-administered by patients
- Soluble tablets or drops
- Sublingual-swallow: keep under the tongue for 1-2 min, then swallow. Contact time with the oral mucosa is critical to the effectiveness of SLIT - if the allergen is immediately swallowed, there are negligible clinical effects.
- Dose of allergen greater than for SCIT (3-300 times higher)
- Administration schedule and amount of allergen vary, depending on the manufacturer
- Amount of allergen given during a course of SLIT is higher than in SCIT: there is a bild-up phase (very rapid), followed by a maintenance phase
Efficacy of SLIT in allergic rhinitis
The magnitude of clinical efficacy ranged from 20-60% reduction of symptoms. House dust mite (HDM) SLIT was less effective, but results were comparable to SCIT. For HDM, duration of treatment seems to be crucial - treatments that lasted longer than 24 months had positive results.
Clinical safety of SCIT vs. SLIT
Safety of SCIT
Systemic reactions occur in 0.05-0.6% of doses administered. Rare fatalities have been reported.
Safety of SLIT
No life-threatening adverse events reported since 1986. Most common adverse effects include oral/sublingual itching, stomachache, and nausea. No increased risk in patients with oral allergy syndrome.
Sublingual immunotherapy is safe during pregnancy, it is also safe when initiated for the first time in pregnancy (study) (Allergy, 2012).
CpG-enhanced immunotherapy
Bacterial DNA contains unmethylated CpG di-nucleotides (CpG motifs) that act as a danger signal to the vertebrate immune system and trigger protective innate and acquired immune responses.
CpGs work through toll-like receptor 9 (TLR-9) to activate monocytes, dendritic cells, B cells, and NK cells. CPGs promote Th1 and inhibit Th2 response (similar to allergen-specific SCIT). TLR9 and MyD88 play central role in protective immunity to malaria http://goo.gl/VVF07
The immune stimulatory activity of bacterial DNA can be mimicked by synthetic oligodeoxynucleotides containing CpG motifs. They can be added to a vaccine or linked to an
antigen to greatly boost the immune response.
Anti-IgE (omalizumab (Xoliar) and immunotherapy
The combination anti-IgE and allergen immunotherapy might offer advantages that
neither method can provide separately. Anti-IgE administered during the induction phase
of immunotherapy might reduce the risk of IgE-mediated anaphylaxis.
References
Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. JACI, 2010.
Related reading
Immunotherapy in Asthma - 11-page Medscape review http://goo.gl/KUAcA
Sublingual immunotherapy is an extremely complex issue in the U.S. AAAAI http://goo.gl/wVOKr
Timothy grass allergy immunotherapy tablets safe and effective in American children with allergic rhinitis http://goo.gl/tsKL4
Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults - it works. http://goo.gl/ePOFG
Epicutaneous allergen administration: is this the future of allergen-specific immunotherapy? Allergy, 2011.
Published: 06/27/2010
Updated: 03/15/2012
Mnemonics: Subcutaneous Immunotherapy (SCIT)
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Published: 05/09/2010
Updated: 09/09/2011
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Mixing allergen extracts with high protease content
Mnemonic
M
Mold
Mite - cockroach has even more proteases than mite
Mixing problems - proteases degarade grass extracts in particular and decrease their potency
References
Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. JACI, 2010.
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Published: 05/09/2010
Updated: 09/09/2011
Venom immunotherapy (VIT)
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).

Mechanisms of allergen-specific immunotherapy (click to enlarge the image).
Extracts
Allergen (venom) vaccine is the recommended term for the therapeutic agent used in allergen immunotherapy. "Vaccine" is used when the therapeutic use of the preparation is
clear. "Extract" is used when the non-therapeutic aspects of the allergen preparation are discussed.
Extracts of honeybee, yellow jacket, white-faced hornet, yellow hornet, and wasp venom are available for skin testing and VIT.
There is no venom extract for fire ant hypersensitivity but a whole-body extract is available.
Tests
Skin prick tests with a concentration in the range of 1.0 to 100 mcg/mL may be performed before intracutaneous (intradermal) tests but are not used by all allergists.
Intracutaneous tests start with a concentration in the range of 0.001 to 0.01 mcg/mL. If
intracutaneous test results at this concentration are negative, the concentration is increased by 10-fold increments until a positive skin test response occurs or a maximum concentration of 1.0 mcg/ mL is reached.
A positive intradermal skin test to insect venom at a concentration of 1.0
mcg/mL or lower is indicative of specific IgE antibodies.
Skin testing with fire ant whole-body extract is indicative of specific IgE antibodies if a positive response occurs at a concentration of 1:100 wt/vol or less by prick method, or 1:1000 wt/vol or less by intradermal method.
If the skin test is negative despite a convincing history of anaphylaxis after
an insect sting, in vitro testing for IgE antibodies or repeat skin testing is recommended.
Venon immunotherapy (VIT)
30-60% of patients with a history of anaphylaxis from an insect sting who have venom-specific IgE antibodies (skin or in vitro testing) will experience a systemic reaction when stung again.
VIT is not necessary in children 16 years of age and younger who have experienced isolated
cutaneous systemic reactions without other systemic manifestations. They only have a 10% chance of having a systemic reaction if stung again, and if one occurs, it is unlikely to be worse
than the initial isolated cutaneous reaction.
VIT in adults who have experienced only cutaneous systemic reaction is controversial but usually recommended.
VIT is extremely effective in reducing the risk of systemic reaction to less than 5%, and sting reactions that occur during VIT are usually milder.
VIT is generally not necessary for patients who have had only a large local reaction because the risk of a systemic reaction is low.
The vast majority of patients who have had a large local reaction do not need to be tested for specific IgE.
How do you define a large local reaction to insect sting?
- increase in size for 24 to 48 hours,
- swelling to more than 10 cm in diameter
- 5 to 10 days to resolve
Patients who have experienced large local reactions often have large local
reactions to subsequent stings, and up to 10% might eventually have a systemic reaction.
What is the difference between a large local reaction and a systemic cutaneous reaction?
Systemic reactions can include a spectrum of manifestations ranging from mild to life-threatening:
- cutaneous reactions (eg, urticaria and angioedema),
- bronchospasm
- large airway obstruction (tongue or throat swelling, laryngeal edema)
- hypotension and shock.
The key feature that distinguishes a systemic cutaneous reaction from a large local reaction is the involvement of parts of the body not contiguous with the site of the sting.
What is the dose of VIT?
VIT injections start weekly, beginning with doses no greater than 0.1 to 1.0 mcg, and increasing to a maintenance dose of 100 mcg of each venom (e.g., 1 mL of a vaccine containing 100 mcg/mL of venom).
The dosage schedule for fire ant immunotherapy is less well defined. A maintenance dose
is 0.5 mL of a 1:100 wt/vol concentration.
The interval between maintenance dose injections can be increased to 4-week intervals during the first year of VIT and to every 6 to 8 weeks during subsequent years.
How long to continue VIT?
VIT should be continued for at least 3 to 5 years. Despite the persistence of a positive skin test response, 80-90% of patients will not have a systemic reaction to an insect sting if VIT is stopped after 3 to 5 years.
Some patients with a history of severe anaphylaxis with shock or loss of consciousness still might be at continued risk for a systemic reaction if VIT is stopped, even after
5 years of immunotherapy.
Patients who have experienced a systemic reaction carry injectable epinephrine (eg, EpiPenTM or TwinJectTM devices) at all times.
Patients who take beta-blocker are at greater risk for anaphylaxis to VIT or a sting. Patients who have stinging insect hypersensitivity should not be prescribed beta-blockers unless absolutely necessary.
References
Stinging Insect Hypersensitivity: A Practice parameter Update. Joint Council of Allergy, Asthma, and Immunology.
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
Mechanisms of allergen-specific immunotherapy (click to enlarge the image).
Extracts
Allergen (venom) vaccine is the recommended term for the therapeutic agent used in allergen immunotherapy. "Vaccine" is used when the therapeutic use of the preparation is
clear. "Extract" is used when the non-therapeutic aspects of the allergen preparation are discussed.
Extracts of honeybee, yellow jacket, white-faced hornet, yellow hornet, and wasp venom are available for skin testing and VIT.
There is no venom extract for fire ant hypersensitivity but a whole-body extract is available.
Tests
Skin prick tests with a concentration in the range of 1.0 to 100 mcg/mL may be performed before intracutaneous (intradermal) tests but are not used by all allergists.
Intracutaneous tests start with a concentration in the range of 0.001 to 0.01 mcg/mL. If
intracutaneous test results at this concentration are negative, the concentration is increased by 10-fold increments until a positive skin test response occurs or a maximum concentration of 1.0 mcg/ mL is reached.
A positive intradermal skin test to insect venom at a concentration of 1.0
mcg/mL or lower is indicative of specific IgE antibodies.
Skin testing with fire ant whole-body extract is indicative of specific IgE antibodies if a positive response occurs at a concentration of 1:100 wt/vol or less by prick method, or 1:1000 wt/vol or less by intradermal method.
If the skin test is negative despite a convincing history of anaphylaxis after
an insect sting, in vitro testing for IgE antibodies or repeat skin testing is recommended.
Venon immunotherapy (VIT)
30-60% of patients with a history of anaphylaxis from an insect sting who have venom-specific IgE antibodies (skin or in vitro testing) will experience a systemic reaction when stung again.
VIT is not necessary in children 16 years of age and younger who have experienced isolated
cutaneous systemic reactions without other systemic manifestations. They only have a 10% chance of having a systemic reaction if stung again, and if one occurs, it is unlikely to be worse
than the initial isolated cutaneous reaction.
VIT in adults who have experienced only cutaneous systemic reaction is controversial but usually recommended.
VIT is extremely effective in reducing the risk of systemic reaction to less than 5%, and sting reactions that occur during VIT are usually milder.
VIT is generally not necessary for patients who have had only a large local reaction because the risk of a systemic reaction is low.
The vast majority of patients who have had a large local reaction do not need to be tested for specific IgE.
How do you define a large local reaction to insect sting?
- increase in size for 24 to 48 hours,
- swelling to more than 10 cm in diameter
- 5 to 10 days to resolve
Patients who have experienced large local reactions often have large local
reactions to subsequent stings, and up to 10% might eventually have a systemic reaction.
What is the difference between a large local reaction and a systemic cutaneous reaction?
Systemic reactions can include a spectrum of manifestations ranging from mild to life-threatening:
- cutaneous reactions (eg, urticaria and angioedema),
- bronchospasm
- large airway obstruction (tongue or throat swelling, laryngeal edema)
- hypotension and shock.
The key feature that distinguishes a systemic cutaneous reaction from a large local reaction is the involvement of parts of the body not contiguous with the site of the sting.
What is the dose of VIT?
VIT injections start weekly, beginning with doses no greater than 0.1 to 1.0 mcg, and increasing to a maintenance dose of 100 mcg of each venom (e.g., 1 mL of a vaccine containing 100 mcg/mL of venom).
The dosage schedule for fire ant immunotherapy is less well defined. A maintenance dose
is 0.5 mL of a 1:100 wt/vol concentration.
The interval between maintenance dose injections can be increased to 4-week intervals during the first year of VIT and to every 6 to 8 weeks during subsequent years.
How long to continue VIT?
VIT should be continued for at least 3 to 5 years. Despite the persistence of a positive skin test response, 80-90% of patients will not have a systemic reaction to an insect sting if VIT is stopped after 3 to 5 years.
Some patients with a history of severe anaphylaxis with shock or loss of consciousness still might be at continued risk for a systemic reaction if VIT is stopped, even after
5 years of immunotherapy.
Patients who have experienced a systemic reaction carry injectable epinephrine (eg, EpiPenTM or TwinJectTM devices) at all times.
Patients who take beta-blocker are at greater risk for anaphylaxis to VIT or a sting. Patients who have stinging insect hypersensitivity should not be prescribed beta-blockers unless absolutely necessary.
References
Stinging Insect Hypersensitivity: A Practice parameter Update. Joint Council of Allergy, Asthma, and Immunology.
Hymenoptera Venom Immunotherapy. Medscape review, 2011.
Stinging Insect Guidelines - 2001 Update by AAAAI and ACAAI. Medscape, 2011.
Published: 10/12/2009
Updated: 06/12/2011
Published: 10/12/2009
Updated: 06/12/2011
How to Write a Subcutaneous Immunotherapy (SCIT) Prescription for Allergic Rhinitis
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 33-year-old AAM is referred to the allergy clinic for symptoms of allergic rhinitis and conjunctivitis. He complains of itchy watery eyes, itchy nose and nasal congestion. These symptoms occur all year but are worse in the spring and summer. He has tried intranasal steroids (INS) and oral antihistamines but does not get a lasting symptom relief and his sleep is impaired. No history of nasal polyps, eczema, food allergies, or systemic reactions to stinging insects.
Past medical history (PMH)
Allergic rhinitis and conjunctivitis.
Medications
Fluticasone (Flonase) 50 mcg/actuation nasal spray QHS, loratidine 10 mg po daily.
Social history (SH)
No tobacco or alcohol use.
Environmental history
Pets in the home: 2 dogs. Flooring: Wall-to-wall carpeting. Air conditioning: Central air. Heating: Forced hot air. Basement: Dry basement. Dust mite controls: Dust mite controls are not in place. Tobacco smoke: no exposure in the home.
Family history (FH)
Mother with asthma and allergic rhinitis. Sister with allergic rhinitis.
Physical examination
VSS
HEENT: External ears normal. Canals clear. TM's normal. Nares normal. Septum midline. Congested pale mucosa, no polyps seen. No drainage or sinus tenderness. Lips, tongue normal. Oropharynx clear.
Neck: supple, no adenopathy
CVS: RRR, normal S1/S2, no m/r/g
Chest: CTA (B)
Extremities: no c/c/e
Skin: color, texture, turgor normal. No rashes or lesions.
What is the most likely diagnosis?
Allergic rhinitis and conjunctivitis.
What tests would you order?
Skin prick test.
What happened?

Figure 1. Skin prick test results (click to enlarge).
The allergen skin test was extremely positive with multiple pseudopods described by the nurse as "pseudopods on pseudopods."
The patient developed conjunctival itching and redness, nasal congestion and discharge during the skin prick test which required administration of epinephrine 0.3 mg IM, loratidine 10 mg and prednisone 40 mg po. His symptoms resolved withing 15 minutes. A second dose of prednisone was prescribed for the next day.
Final diagnosis
Allergic rhinitis and conjunctivitis.
What treatment options would you suggest for long-term control of his symptoms?
Subcutaneous immunotherapy (SCIT).
Risks and benefits of subcutaneous immunotherapy (SCIT) for allergic rhinitis were discussed and the patient opted to start therapy.
How would you write a prescription for SCIT?

Figure 2. SCIT Instructions (click to enlarge).
Please see the SCIT instructions above. Multiallergic patiens may need 3 vials of mixed immunotherapy extracts labeled A, B and C.
Please see the table for protease activity in the SCIT instructions above. High protease activity extracts cannot mix with the low protease activity extracts because they degrade them. For example, mold and dust mite extracts can only be mixed together (and with ragweed). Ragweed extract can be mixed with all other extracts.
You must have in mind the total volume of each vial when writing a SCIT prescription. In this case, the total volume of each vial is 5 ml. The extracts can be mixed up to 5 ml in each vial. The rest of the volume is made up with diluent.
You can use a Microsoft Excel Spreadsheet or similar software to calculate the sum of the volumes for different extracts and the volume of diluent needed.

Figure 3. SCIT prescription. (click to enlarge).
You need figures 1, 2 and 3 in front of you when writing a prescription for each patient.
Figure 1 (skin prick test) determines what will be put in the mix.
Figure 2 (reminder "cheat sheet") determines how the extracts will be mixed in each vial.
Figure 3 is the actual SCIT prescription.
It is advisable to have all three figures in front of you when writing SCIT prescriptions, at least initially.
Let's look at vial A. Our patient is allergic to almost all allergens tested. We will mix in vial A the following allergens:
- Grass Mix #7, 0.4 ml
- Bermuda, 0.5 ml
- Creighton Tree Mix (or local mix for the particular area), 1.5 ml
- Short Ragweed 1.0 ml
The amount of diluent needed to constitute the vial to a total of 5 ml is 0.3 ml.
Let's look at vial B. We will mix in vial B the following allergens (all with high protease activity):
- Mite Mix, 0.3 ml
- Cockroach, 0.5 ml
- Mold Mix AHP, 2.0 ml
- Minor Mold Mix, 2.0 ml
All the allergens above have high protease activity and can be mixed together. They should not be mixed with low protease activity extracts.
The amount of diluent needed to constitute the vial to a total of 5 ml is 0.2 ml.
Let's look at vial C. We will mix in vial C the following allergens:
- Cat Hair, 2.0 ml
- Dog Hair, 2.0 ml
Cat and dog hair require relatively larger volumes (2 ml each) compared to other allergens. Therefore, relatively fewer allergens are mixed in vial C.
The amount of diluent needed to constitute the vial to a total of 5 ml is 1.0 ml.
When a patient is "multiallergic," the goal should be to maximize the dose of extracts for which we have the best evidence for effectiveness: grass, ragweed and dust mite. Allergen immunotherapy with dog hair is generally less effective than the one with cat hair.
It is difficult for children to tolerate 3 SCIT injection, therefore every effort should be made to combine the extracts in 1-2 vials.
What is the starting dose of SCIT?
The patient had a hypersensitivity reaction during the skin prick testing, therefore we decided to start with the lowest dose listed in figure 3 at a dilution of 1:10,000. The dose will be administered weekly.



Vials A, B and C - mixed.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Talking Points on Sublingual Immunotherapy (SLIT) for Physicians Practicing in the United States. ACAAI.
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 33-year-old AAM is referred to the allergy clinic for symptoms of allergic rhinitis and conjunctivitis. He complains of itchy watery eyes, itchy nose and nasal congestion. These symptoms occur all year but are worse in the spring and summer. He has tried intranasal steroids (INS) and oral antihistamines but does not get a lasting symptom relief and his sleep is impaired. No history of nasal polyps, eczema, food allergies, or systemic reactions to stinging insects.
Past medical history (PMH)
Allergic rhinitis and conjunctivitis.
Medications
Fluticasone (Flonase) 50 mcg/actuation nasal spray QHS, loratidine 10 mg po daily.
Social history (SH)
No tobacco or alcohol use.
Environmental history
Pets in the home: 2 dogs. Flooring: Wall-to-wall carpeting. Air conditioning: Central air. Heating: Forced hot air. Basement: Dry basement. Dust mite controls: Dust mite controls are not in place. Tobacco smoke: no exposure in the home.
Family history (FH)
Mother with asthma and allergic rhinitis. Sister with allergic rhinitis.
Physical examination
VSS
HEENT: External ears normal. Canals clear. TM's normal. Nares normal. Septum midline. Congested pale mucosa, no polyps seen. No drainage or sinus tenderness. Lips, tongue normal. Oropharynx clear.
Neck: supple, no adenopathy
CVS: RRR, normal S1/S2, no m/r/g
Chest: CTA (B)
Extremities: no c/c/e
Skin: color, texture, turgor normal. No rashes or lesions.
What is the most likely diagnosis?
Allergic rhinitis and conjunctivitis.
What tests would you order?
Skin prick test.
What happened?

Figure 1. Skin prick test results (click to enlarge).
The allergen skin test was extremely positive with multiple pseudopods described by the nurse as "pseudopods on pseudopods."
The patient developed conjunctival itching and redness, nasal congestion and discharge during the skin prick test which required administration of epinephrine 0.3 mg IM, loratidine 10 mg and prednisone 40 mg po. His symptoms resolved withing 15 minutes. A second dose of prednisone was prescribed for the next day.
Final diagnosis
Allergic rhinitis and conjunctivitis.
What treatment options would you suggest for long-term control of his symptoms?
Subcutaneous immunotherapy (SCIT).
Risks and benefits of subcutaneous immunotherapy (SCIT) for allergic rhinitis were discussed and the patient opted to start therapy.
How would you write a prescription for SCIT?

Figure 2. SCIT Instructions (click to enlarge).
Please see the SCIT instructions above. Multiallergic patiens may need 3 vials of mixed immunotherapy extracts labeled A, B and C.
Please see the table for protease activity in the SCIT instructions above. High protease activity extracts cannot mix with the low protease activity extracts because they degrade them. For example, mold and dust mite extracts can only be mixed together (and with ragweed). Ragweed extract can be mixed with all other extracts.
You must have in mind the total volume of each vial when writing a SCIT prescription. In this case, the total volume of each vial is 5 ml. The extracts can be mixed up to 5 ml in each vial. The rest of the volume is made up with diluent.
You can use a Microsoft Excel Spreadsheet or similar software to calculate the sum of the volumes for different extracts and the volume of diluent needed.

Figure 3. SCIT prescription. (click to enlarge).
You need figures 1, 2 and 3 in front of you when writing a prescription for each patient.
Figure 1 (skin prick test) determines what will be put in the mix.
Figure 2 (reminder "cheat sheet") determines how the extracts will be mixed in each vial.
Figure 3 is the actual SCIT prescription.
It is advisable to have all three figures in front of you when writing SCIT prescriptions, at least initially.
Let's look at vial A. Our patient is allergic to almost all allergens tested. We will mix in vial A the following allergens:
- Grass Mix #7, 0.4 ml
- Bermuda, 0.5 ml
- Creighton Tree Mix (or local mix for the particular area), 1.5 ml
- Short Ragweed 1.0 ml
The amount of diluent needed to constitute the vial to a total of 5 ml is 0.3 ml.
Let's look at vial B. We will mix in vial B the following allergens (all with high protease activity):
- Mite Mix, 0.3 ml
- Cockroach, 0.5 ml
- Mold Mix AHP, 2.0 ml
- Minor Mold Mix, 2.0 ml
All the allergens above have high protease activity and can be mixed together. They should not be mixed with low protease activity extracts.
Mixing allergen extracts with high protease content
Mnemonic
M
Mold
Mite - cockroach has even more proteases than mite
Mixing problems - proteases degarade grass extracts in particular and decrease their potency
The amount of diluent needed to constitute the vial to a total of 5 ml is 0.2 ml.
Let's look at vial C. We will mix in vial C the following allergens:
- Cat Hair, 2.0 ml
- Dog Hair, 2.0 ml
Cat and dog hair require relatively larger volumes (2 ml each) compared to other allergens. Therefore, relatively fewer allergens are mixed in vial C.
The amount of diluent needed to constitute the vial to a total of 5 ml is 1.0 ml.
When a patient is "multiallergic," the goal should be to maximize the dose of extracts for which we have the best evidence for effectiveness: grass, ragweed and dust mite. Allergen immunotherapy with dog hair is generally less effective than the one with cat hair.
It is difficult for children to tolerate 3 SCIT injection, therefore every effort should be made to combine the extracts in 1-2 vials.
What is the starting dose of SCIT?
The patient had a hypersensitivity reaction during the skin prick testing, therefore we decided to start with the lowest dose listed in figure 3 at a dilution of 1:10,000. The dose will be administered weekly.



Vials A, B and C - mixed.
What are the 4 standardized allergen extracts?
(A) Dog
(B) Trees
(C) Cat
(D) Molds
(E) Dust Mite
(F) Grass
(G) Ragweed
The 4 standardized extracts are Cat, Dust Mite, Grass and Ragweed.
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012). Allergists should provide an EpiPen prescriptions to all patients on SCIT.
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012). Allergists should provide an EpiPen prescriptions to all patients on SCIT.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Talking Points on Sublingual Immunotherapy (SLIT) for Physicians Practicing in the United States. ACAAI.
Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. JACI, 2010.
SCIT ("allergy shots") is at least as potent as pharmacotherapy in controlling the symptoms of allergic rhintis as early as the first season of therapy. JACI, 2011.
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Video
Immunotherapy Rx, Part 1 and 2, Jay Portnoy, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, 2009.
Immunotherapy. Linda Cox, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, Feb 4, 2009.
Allergy shots by Dr. Y. Patel.
Patient Information
What is immunotherapy and how does it work? AAAAI, 2006.
Published: 02/24/2009
Updated: 09/26/2011
Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes (2011) http://goo.gl/xHYjG
Video
Immunotherapy Rx, Part 1 and 2, Jay Portnoy, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, 2009.
Immunotherapy. Linda Cox, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, Feb 4, 2009.
Allergy shots by Dr. Y. Patel.
Patient Information
What is immunotherapy and how does it work? AAAAI, 2006.
Published: 02/24/2009
Updated: 09/26/2011
Anaphylactic Reaction to Subcutaneous Immunotherapy in a Patient with Asthma: How Do You Change the Dose?
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 16-year-old CF is on a maintenance dose of subcutaneous immunotherapy (SCIT) for allergic rhinitis and asthma. She receives the injections by in her primary care physician office in a different town and the allergy clinic prepares the mixtures. The immunotherapy prescription consists of trees, grasses, dust mite, cat, dog and cockroach. She has been on a maintenance dose (injections given every 3 weeks) for 1.5 years and has had several episodes of large local reaction. Three dose ago, she had an URI with mild fever but still went for her "allergy shot." There was a new nurse who administered the injection "higher than usual" in the area of the deltoid muscle. Within 2-3 minutes, she developed shortness of breath, wheezing and desaturation in the 80s. She was treated with an EpiPen, prednisone, loratidine and albuterol inhalation. Her condition improved and after an evaluation in th ED, she was discharged home.
Past medical history (PMH)
Allergic rhinitis and conjunctivitis, asthma.
Medications
Flovent 110 mcg INH bid, Fluticasone (Flonase) 50 mcg/actuation nasal spray QHS, loratidine 10 mg po daily.
Social history (SH)
No tobacco or alcohol use.
Family history (FH)
Mother with asthma and allergic rhinitis.
Physical examination
Normal.
What is the most likely diagnosis?
Anaphylactic reaction to SCIT.
What is the most likely reason for the anaphylactic reaction?
Patients with asthma are at higher risk for fatal anaphylactic reactions. Virus infections and febrile conditions create and inflammatory evnironment that could have contributed to her anaphylactic reaction. The injection in the area of the deltoid muscle could be inadvertently administered IM instead of SC, especially of the personnel is inexperienced.
How would you change the SCIT dose?
She currently receives 0.4 ml of the 1:1 dilution. SCIT dose was decreased by "two dilutions" - to 0.4 ml of dilution 1:100.
She was advised to receive the next SCIT dose in our office and to consider receiving the following doses at an allergy clinic in her home town rather than at her PCP's office.
Final diagnosis
Anaphylcatic reaction to SCIT.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 16-year-old CF is on a maintenance dose of subcutaneous immunotherapy (SCIT) for allergic rhinitis and asthma. She receives the injections by in her primary care physician office in a different town and the allergy clinic prepares the mixtures. The immunotherapy prescription consists of trees, grasses, dust mite, cat, dog and cockroach. She has been on a maintenance dose (injections given every 3 weeks) for 1.5 years and has had several episodes of large local reaction. Three dose ago, she had an URI with mild fever but still went for her "allergy shot." There was a new nurse who administered the injection "higher than usual" in the area of the deltoid muscle. Within 2-3 minutes, she developed shortness of breath, wheezing and desaturation in the 80s. She was treated with an EpiPen, prednisone, loratidine and albuterol inhalation. Her condition improved and after an evaluation in th ED, she was discharged home.
Past medical history (PMH)
Allergic rhinitis and conjunctivitis, asthma.
Medications
Flovent 110 mcg INH bid, Fluticasone (Flonase) 50 mcg/actuation nasal spray QHS, loratidine 10 mg po daily.
Social history (SH)
No tobacco or alcohol use.
Family history (FH)
Mother with asthma and allergic rhinitis.
Physical examination
Normal.
What is the most likely diagnosis?
Anaphylactic reaction to SCIT.
What is the most likely reason for the anaphylactic reaction?
Patients with asthma are at higher risk for fatal anaphylactic reactions. Virus infections and febrile conditions create and inflammatory evnironment that could have contributed to her anaphylactic reaction. The injection in the area of the deltoid muscle could be inadvertently administered IM instead of SC, especially of the personnel is inexperienced.
How would you change the SCIT dose?
She currently receives 0.4 ml of the 1:1 dilution. SCIT dose was decreased by "two dilutions" - to 0.4 ml of dilution 1:100.
She was advised to receive the next SCIT dose in our office and to consider receiving the following doses at an allergy clinic in her home town rather than at her PCP's office.
Final diagnosis
Anaphylcatic reaction to SCIT.
What are the 4 standardized allergen extracts?
(A) Dog
(B) Trees
(C) Cat
(D) Molds
(E) Dust Mite
(F) Grass
(G) Ragweed
The 4 standardized extracts are Cat, Dust Mite, Grass and Ragweed.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Treatment of anaphylactic reactions due to immunotherapy. AAAAI - Ask the Expert, 2011.
Published: 03/20/2009
Updated: 01/20/2011
Published: 03/20/2009
Updated: 01/20/2011
Procedure Guide: Subcutaneous Immunotherapy
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012). The type of extracts used for subcutaneous immunotherapy depends on the reactivity to a particular allergen on the skin prick testing.

Diagram of skin prick testing

Skin test sheet. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
The skin prick allergens are listed on the sheet in the order they appear in the environment during the year and can be remembered with the mnemonic:
How the allergens change during the season: mnemonic TGR MI DC/DC ("a Tiger with an MI went to DC")
"There's spring time, where you have the tree pollen. Summer time, where you have the grass pollens. And then there's the fall time when you have the weed pollen.”
This sequence is remembered by the mnemonic TGR MI DC/DC ("a Tiger with an MI went to DC").
Pollen calendar: TGR MI DC/DC
Tree pollens
Grass pollens
Ragweed and weed pollen
Mold spores
Indoor allergens - DC/DC - Dog/Cat and Dust mite/Cockroach
Pollen-producing plants (weeds and trees) in Omaha, Nebraska
References:
Characteristics of allergic sensitization among asthmatic adults older than 55 years: results from the National Health Allergy Season Year Round. WTOC-TV Savannah.
Interactive Allergy Map by Greer Labs. Click your state to find region-specific, common airborne allergens there.
‘Botanical sexism’ blamed for making life miserable for allergy sufferers as male trees fill city skies with pollen http://goo.gl/cx5tH


Immunotherapy Preparation. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
Molds include AAHP (Alternaria, Aspergillus, Hormodendrum, Penicillium) and Minor Mold Mix.
Many patients require 2 vials of extracts. Each vial contains 10 ml of extract solution. If the total amount of allergen extract is less than 10 ml, the rest of vial is filled with diluent to a total of 10 ml.
Animal extracts (cat, dog) are not mixed with "other animals" such as cockroach, mite, molds. Molds include AAHP (Alternaria, Aspergillus, Hormodendrum, Penicillium) and Minor Mold Mix.

Immunotherapy order sheet. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012). The type of extracts used for subcutaneous immunotherapy depends on the reactivity to a particular allergen on the skin prick testing.

Diagram of skin prick testing

Skin test sheet. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
The skin prick allergens are listed on the sheet in the order they appear in the environment during the year and can be remembered with the mnemonic:
How the allergens change during the season: mnemonic TGR MI DC/DC ("a Tiger with an MI went to DC")
"There's spring time, where you have the tree pollen. Summer time, where you have the grass pollens. And then there's the fall time when you have the weed pollen.”
This sequence is remembered by the mnemonic TGR MI DC/DC ("a Tiger with an MI went to DC").
Pollen calendar: TGR MI DC/DC
Tree pollens
Grass pollens
Ragweed and weed pollen
Mold spores
Indoor allergens - DC/DC - Dog/Cat and Dust mite/Cockroach
Pollen-producing plants (weeds and trees) in Omaha, Nebraska
References:
Characteristics of allergic sensitization among asthmatic adults older than 55 years: results from the National Health Allergy Season Year Round. WTOC-TV Savannah.
Interactive Allergy Map by Greer Labs. Click your state to find region-specific, common airborne allergens there.
‘Botanical sexism’ blamed for making life miserable for allergy sufferers as male trees fill city skies with pollen http://goo.gl/cx5tH


Immunotherapy Preparation. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
Molds include AAHP (Alternaria, Aspergillus, Hormodendrum, Penicillium) and Minor Mold Mix.
Many patients require 2 vials of extracts. Each vial contains 10 ml of extract solution. If the total amount of allergen extract is less than 10 ml, the rest of vial is filled with diluent to a total of 10 ml.
Animal extracts (cat, dog) are not mixed with "other animals" such as cockroach, mite, molds. Molds include AAHP (Alternaria, Aspergillus, Hormodendrum, Penicillium) and Minor Mold Mix.

Immunotherapy order sheet. Image source: Dr. Stokes, Creighton University Division of Allergy & Immunology, used with permission.
Mixing allergen extracts with high protease content
Mnemonic
M
Mold
Mite - cockroach has even more proteases than mite
Mixing problems - proteases degarade grass extracts in particular and decrease their potency
Sublingual immunotherapy
Sublingual immunotherapy induces similar immunologic alterations as subcutaneous immunotherapy, although to a lesser degree.
Omalizumab
The combination of omalizumab with allergen subcutaneous immunotherapy can enhance clinical efficacy.
Oral Immunotherapy
Sublingual immunotherapy induces similar immunologic alterations as subcutaneous immunotherapy, although to a lesser degree.
Currently the only product approved for clinical use by European regulators is a tablet containing timothy grass extract. There are no products approved for oral immunotherapy in the US.
Optimal duration of sublingual therapy is not defined. The NEJM published a review of SLIT in 2008.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Allergen-Specific Immunotherapy of Allergy and Asthma: Current and Future Trends. François Spertini; Christophe Reymond; Annette Leimgruber. Expert Review of Respiratory Medicine, Medscape, 03/2009.
Video
Immunotherapy Rx, Part 1 and 2, Jay Portnoy, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, 2009.
Immunotherapy. Linda Cox, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, Feb 4, 2009.
Patient Information
What is immunotherapy and how does it work? AAAAI, 2006.
Published: 07/03/2008
Updated: 08/15/2010
Sublingual immunotherapy induces similar immunologic alterations as subcutaneous immunotherapy, although to a lesser degree.
Omalizumab
The combination of omalizumab with allergen subcutaneous immunotherapy can enhance clinical efficacy.
Oral Immunotherapy
Sublingual immunotherapy induces similar immunologic alterations as subcutaneous immunotherapy, although to a lesser degree.
Currently the only product approved for clinical use by European regulators is a tablet containing timothy grass extract. There are no products approved for oral immunotherapy in the US.
Optimal duration of sublingual therapy is not defined. The NEJM published a review of SLIT in 2008.
What are the 4 standardized allergen extracts?
(A) Dog
(B) Trees
(C) Cat
(D) Molds
(E) Dust Mite
(F) Grass
(G) Ragweed
The 4 standardized extracts are Cat, Dust Mite, Grass and Ragweed.
References
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Allergen Immunotherapy. AFP, 2004.
Allergy Immunotherapy for Primary Care Physicians. J . Stokes , T . Casale. The American Journal of Medicine , Volume 119 , Issue 10 , Pages 820 - 823 (2006). Link via MDConsult.
Position Statement on the Administration of Immunotherapy Outside of the Prescribing Allergist Facility. ACAAI.
Allergen injection immunotherapy. John M Weiner. MJA 2006; 185 (4): 234.
Use of Immunotherapy in a Primary Care Office. AFP, 1998.
Advances in upper airway diseases and allergen immunotherapy in 2007. Saltoun C, Avila PC. J Allergy Clin Immunol. 2008 Aug 9.
Sublingual Immunotherapy. Anthony J. Frew. NEJM, Volume 358:2259-2264, May 22, 2008.
Allergen-Specific Immunotherapy of Allergy and Asthma: Current and Future Trends. François Spertini; Christophe Reymond; Annette Leimgruber. Expert Review of Respiratory Medicine, Medscape, 03/2009.
Video
Immunotherapy Rx, Part 1 and 2, Jay Portnoy, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, 2009.
Immunotherapy. Linda Cox, MD. Conferences Online For Allergy, Children's Mercy Hospitals & Clinics, Feb 4, 2009.
Allergy shots by Dr. Y. Patel.
Patient Information
What is immunotherapy and how does it work? AAAAI, 2006.
Published: 07/03/2008
Updated: 08/15/2010
Cough Due to GERD in a Patient with Allergic Rhinitis
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 37-year-old female with allergic rhinitis on immunotherapy comes to the allergy clinic with symptoms of dry cough and heartburn for 3 weeks. She has had this type of cough off and on for the last 2 years. The patient was treated with Prilosec 1.5 years ago with good effect but she stopped the medication after 4 months due to "bloating." She would like to receive her immunotherapy shot today.
Past medical history (PMH)
Alelrgic rhinitis.
Medications
Flonase (fluticasone), Zyrtec (cetirizine).
Skin testing
Two years ago.
Pulmonary function tests (PFTs)
Normal, 1 year ago
Immunotherapy
Started 5 months ago, last dose was 4 weeks ago. Grass and tree extracts were used.
Pets
Dog and cat, the family also has horses and chicken.
Physical examination
Normal.
Spirometry today
Normal.
What is the reason for the cough?
Most likely GERD.
What would you do?
The patient was prescribed Nexium and advised to return to the clinic in 2 weeks.
Would you give the scheduled immunotherapy injection today?
It is generally advisable to postpone the administration of the immunotherapy injection until acute symptoms resolve. If the patient's symptoms worsen after the injection, it would be unclear what the cause was: GERD or anaphylaxis.
Which animal is "worse" for pollen-allergic patients - cat or dog?
A typical cat spends most of his/her life indoors and although cat hair has a higher allergic potential than dog hair, cats are less important than dogs for patients with pollen-related allergies. Many dogs, on the other hand, roam outside every day and get back home with "a suit of tree, grass, weed and mold."
Final diagnosis
GERD-related cough.

Differential diagnosis of cough, a simple mnemonic is GREAT BAD CAT TOM. Click here to enlarge the image: (GERD (reflux), Laryngopharyngeal Reflux (LPR), Rhinitis (both allergic and non-allergic) with post-nasal drip (upper airway cough syndrome), Embolism, e.g. PE in adults, Asthma, TB (tuberculosis), Bronchitis, pneumonia, pertussis, Aspiration, e.g foreign body in children, Drugs, e.g. ACE inhibitor, CF in children, Cardiogenic, e.g. mitral stenosis in adults, Achalasia in adults, Thyroid enlargement, e.g. goiter, "Thoughts" (psychogenic), Other causes, Malignancy, e.g. lung cancer in adults).
What did we learn from this case?
GERD is a common cause of cough in patients with allergy in the absence of asthma. It is prudent to await the resolution of acute symptoms before immunotherapy is resumed.
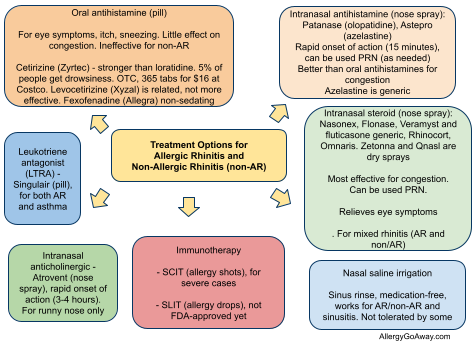
Treatment Options for Allergic Rhinitis (click to enlarge the image).
Published: 07/03/2008
Updated: 01/11/2012
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at LSU (Shreveport) Department of Allergy and Immunology
A 37-year-old female with allergic rhinitis on immunotherapy comes to the allergy clinic with symptoms of dry cough and heartburn for 3 weeks. She has had this type of cough off and on for the last 2 years. The patient was treated with Prilosec 1.5 years ago with good effect but she stopped the medication after 4 months due to "bloating." She would like to receive her immunotherapy shot today.
Past medical history (PMH)
Alelrgic rhinitis.
Medications
Flonase (fluticasone), Zyrtec (cetirizine).
Skin testing
Two years ago.
Pulmonary function tests (PFTs)
Normal, 1 year ago
Immunotherapy
Started 5 months ago, last dose was 4 weeks ago. Grass and tree extracts were used.
Pets
Dog and cat, the family also has horses and chicken.
Physical examination
Normal.
Spirometry today
Normal.
What is the reason for the cough?
Most likely GERD.
What would you do?
The patient was prescribed Nexium and advised to return to the clinic in 2 weeks.
Would you give the scheduled immunotherapy injection today?
It is generally advisable to postpone the administration of the immunotherapy injection until acute symptoms resolve. If the patient's symptoms worsen after the injection, it would be unclear what the cause was: GERD or anaphylaxis.
Which animal is "worse" for pollen-allergic patients - cat or dog?
A typical cat spends most of his/her life indoors and although cat hair has a higher allergic potential than dog hair, cats are less important than dogs for patients with pollen-related allergies. Many dogs, on the other hand, roam outside every day and get back home with "a suit of tree, grass, weed and mold."
Final diagnosis
GERD-related cough.
Differential diagnosis of cough, a simple mnemonic is GREAT BAD CAT TOM. Click here to enlarge the image: (GERD (reflux), Laryngopharyngeal Reflux (LPR), Rhinitis (both allergic and non-allergic) with post-nasal drip (upper airway cough syndrome), Embolism, e.g. PE in adults, Asthma, TB (tuberculosis), Bronchitis, pneumonia, pertussis, Aspiration, e.g foreign body in children, Drugs, e.g. ACE inhibitor, CF in children, Cardiogenic, e.g. mitral stenosis in adults, Achalasia in adults, Thyroid enlargement, e.g. goiter, "Thoughts" (psychogenic), Other causes, Malignancy, e.g. lung cancer in adults).
What did we learn from this case?
GERD is a common cause of cough in patients with allergy in the absence of asthma. It is prudent to await the resolution of acute symptoms before immunotherapy is resumed.
Treatment Options for Allergic Rhinitis (click to enlarge the image).
Related reading
In patients with asthma and chronic productive cough, polymorphonuclear (PMN) neutrophil leukocytes in sputum suggest:
(A) infection
(B) GERD
(C) presence of a foreign body
(D) exercise-induced asthma
(E) extrinsic asthma
Correct answers: A, B, C
PPIs Not Recommended for Routine Treatment of Adult Asthma - in patients with "silent" GERD. Medscape, 2011.
Insufficient evidence to recommend empirical use of PPIs for routine treatment of asthma. Arch Intern Med. 2011;171(7):620-629.
Updated: 01/11/2012
Anaphylactic reaction to subcutaneous immunotherapy: what to do?
Author: V. Dimov, M.D., Allergist/Immunologist and Assistant Professor at University of Chicago
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at NSU
A 31-year-old Caucasian male has been on subcutaneous immunotherapy for allergic rhinitis for 3 months. The subcutaneous immunotherapy (SCIT) consists of 3 injections with extracts of grasses, trees, weeds (vial A), dust mite, molds (vial B), cat and ragweed (vial C). His maintenance dose goal is 0.5 ml.
The SCIT dose was gradually increased with weekly injections and the dose he received last week was 0.3 ml. The patient reports large local reactions which started at the level of 0.1 ml and increased progressively as the dose increased to 0.2 ml.
During the last visit, the size of the local reaction was 30 x 30 mm in terms of swelling. He has no history of prior systemic reactions to SCIT.
Past medical history (PMH)
Allergic rhinitis. He has a remote history of mild asthma, which has been asymptomatic for years and he used only occasionally a prn albuterol inhaler in the remote past.
Medications
Benadryl PRN, Flonase (fluticasone) nasal spray daily
What happened?
The patient received three injections of immunotherapy today at 10:50 and within two to three minutes of the injection, he started to complain of feeling that his throat was closing, dry cough and itchy eyes. He was evaluated immediately by the nurses and his allergist.
What is the most likely diagnosis?
He was found to have an anaphylactic reaction to the subcutaneous immunotherapy.
What treatment would you suggest?
He was given a dose of epineprine 0.3 mg IM at 10:51 and Alavert 10 mg po dissolvable tablet at 10:52. At that time, his blood pressure was 140/55, heart rate was 112, and his pulse-oximetry was 93% on room air.
At 11:00, he was given 40 mg of prednisone po x 1.
What happened next?
The patient reported that his throat sensation was better; however, his pulse-oximetry was noted to be in the range of 90% and on physical examination, he developed diffuse bilateral expiratory wheezing. The physical examination was also remarkable for conjunctival injection and development of swelling around the injection site on both arms with large, local reaction in the range of 8 to 9 cm on the left arm with wheals and satellite wheals around the injection sites.
What treatment would you suggest next?
He was treated with albuterol four puffs at 11:15. At 11:20, he reported improvement in his throat sensation and shortness of breath. His pulse-oximetry was 96%; blood pressure was 130/80.
At 11:30, the patient reportedly returned to baseline in terms of his symptoms. On physical examination, he had no more wheezing.
He was given a prescription for prednisone 40 mg po daily for three days and loratadine 10 mg po daily for seven days.
How would you change the immunotherapy prescription?
His dose of immunotherapy was returned to the dose two steps before the current one, which was 0.1 ml and he is to stay on this dose for two months.
The patient was discharged from the clinic at 12:50, two hours after the event. He is on prednisone, which should prevent any symptoms of late reaction.
Final diagnosis
Anaphylactic reaction to subcutaneous immunotherapy
Summary

Anaphylaxis mind map diagram.
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
During a retrospective chart review of 388 patients, the rate of systemic reactions during subcutaneous immunotherapy was 0.28% per injection and 7.4% per patient. It was concerning that 48% of the systemic reactions occurred more than 30 minutes after the injection and many of these reactions required epinephrine.
This study was unable to identify risk factors that predict the reactions. Gender, phase (build-up versus maintenance), asthma, angiotensin-converting enzyme inhibitors, beta-blockers, initial skin-prick test size, or allergen type did not increase the odds of a systemic reaction.
Skin prick testing (SPT) on beta-blockers was safe in 199 patients in a 2012 study (http://goo.gl/3vGSl). However, incidence of systemic reactions is 1:250 with SPT.
Mnemonics for anaphylaxis
Clinical features of anaphylaxis: S ECG
Skin, 90%
Expiratory wheezing and other respiratory symptoms, 70%
Cardiovascular, 40%
GI and oral, 24%
Risk factors for anaphylaxis due to immunotherapy include: OH BEA
Observation - insufficient, following injection
High allergen dose
Beta-blockers
Errors in administration
Asthma, poorly controlled
Drugs for acute management of anaphylaxis: EASI
Epinephrine IM
Antihistamines PO, IM
Steroids PO, IM, IV
Inhaled b2-agonists, if wheezing. IV fluids if hypotension
Epinephrine (adrenaline) is the first-line the treatment of anaphylaxis. Adult intramuscular dose is 0.3 to 0.5 ml of 1:1,000 concentration. This should be given in the lateral aspect of the thigh by intramuscular injection. The dose can be repeated every 5 to 15 minutes, depending upon the response, for 3-4 doses. The same is true for children except the dose is 0.01 mg per kg (AAAAI Ask the Expert, 2012).
References
Allergen immunotherapy safety: Characterizing systemic reactions and identifying risk factors. Rank, Mathew A.; Oslie, Corrine L.; Krogman, Jennifer L.; Park, Miguel A.; Li, James T. Allergy and Asthma Proceedings, Volume 29, Number 4, 7/8 2008 , pp. 400-405(6).
Evaluation of near-fatal reactions to allergen immunotherapy injections. Amin HS, Liss GM, Bernstein DI. J Allergy Clin Immunol. 2006 Jan;117(1):169-75.
Anaphylactic reactions during immunotherapy. Rezvani M, Bernstein DI. Immunol Allergy Clin North Am. 2007 May;27(2):295-307, viii.
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Anaphylaxis: A Short Review
Rate of systemic reactions during subcutaneous immunotherapy: 0.28% per injection
Mnemonics: Anaphylaxis
Mind Maps: Anaphylaxis
Reviewer: S. Randhawa, M.D., Allergist/Immunologist and Assistant Professor at NSU
A 31-year-old Caucasian male has been on subcutaneous immunotherapy for allergic rhinitis for 3 months. The subcutaneous immunotherapy (SCIT) consists of 3 injections with extracts of grasses, trees, weeds (vial A), dust mite, molds (vial B), cat and ragweed (vial C). His maintenance dose goal is 0.5 ml.
The SCIT dose was gradually increased with weekly injections and the dose he received last week was 0.3 ml. The patient reports large local reactions which started at the level of 0.1 ml and increased progressively as the dose increased to 0.2 ml.
During the last visit, the size of the local reaction was 30 x 30 mm in terms of swelling. He has no history of prior systemic reactions to SCIT.
Past medical history (PMH)
Allergic rhinitis. He has a remote history of mild asthma, which has been asymptomatic for years and he used only occasionally a prn albuterol inhaler in the remote past.
Medications
Benadryl PRN, Flonase (fluticasone) nasal spray daily
What happened?
The patient received three injections of immunotherapy today at 10:50 and within two to three minutes of the injection, he started to complain of feeling that his throat was closing, dry cough and itchy eyes. He was evaluated immediately by the nurses and his allergist.
What is the most likely diagnosis?
He was found to have an anaphylactic reaction to the subcutaneous immunotherapy.
What treatment would you suggest?
He was given a dose of epineprine 0.3 mg IM at 10:51 and Alavert 10 mg po dissolvable tablet at 10:52. At that time, his blood pressure was 140/55, heart rate was 112, and his pulse-oximetry was 93% on room air.
At 11:00, he was given 40 mg of prednisone po x 1.
What happened next?
The patient reported that his throat sensation was better; however, his pulse-oximetry was noted to be in the range of 90% and on physical examination, he developed diffuse bilateral expiratory wheezing. The physical examination was also remarkable for conjunctival injection and development of swelling around the injection site on both arms with large, local reaction in the range of 8 to 9 cm on the left arm with wheals and satellite wheals around the injection sites.
What treatment would you suggest next?
He was treated with albuterol four puffs at 11:15. At 11:20, he reported improvement in his throat sensation and shortness of breath. His pulse-oximetry was 96%; blood pressure was 130/80.
At 11:30, the patient reportedly returned to baseline in terms of his symptoms. On physical examination, he had no more wheezing.
He was given a prescription for prednisone 40 mg po daily for three days and loratadine 10 mg po daily for seven days.
How would you change the immunotherapy prescription?
His dose of immunotherapy was returned to the dose two steps before the current one, which was 0.1 ml and he is to stay on this dose for two months.
The patient was discharged from the clinic at 12:50, two hours after the event. He is on prednisone, which should prevent any symptoms of late reaction.
Final diagnosis
Anaphylactic reaction to subcutaneous immunotherapy
Summary

Anaphylaxis mind map diagram.
Allergen immunotherapy was introduced by Leonard Noon 100 years ago and is the only disease-modifying treatment for allergic individuals (Allergy, 2012).
During a retrospective chart review of 388 patients, the rate of systemic reactions during subcutaneous immunotherapy was 0.28% per injection and 7.4% per patient. It was concerning that 48% of the systemic reactions occurred more than 30 minutes after the injection and many of these reactions required epinephrine.
This study was unable to identify risk factors that predict the reactions. Gender, phase (build-up versus maintenance), asthma, angiotensin-converting enzyme inhibitors, beta-blockers, initial skin-prick test size, or allergen type did not increase the odds of a systemic reaction.
Skin prick testing (SPT) on beta-blockers was safe in 199 patients in a 2012 study (http://goo.gl/3vGSl). However, incidence of systemic reactions is 1:250 with SPT.
Mnemonics for anaphylaxis
Clinical features of anaphylaxis: S ECG
Skin, 90%
Expiratory wheezing and other respiratory symptoms, 70%
Cardiovascular, 40%
GI and oral, 24%
Risk factors for anaphylaxis due to immunotherapy include: OH BEA
Observation - insufficient, following injection
High allergen dose
Beta-blockers
Errors in administration
Asthma, poorly controlled
Drugs for acute management of anaphylaxis: EASI
Epinephrine IM
Antihistamines PO, IM
Steroids PO, IM, IV
Inhaled b2-agonists, if wheezing. IV fluids if hypotension
Epinephrine (adrenaline) is the first-line the treatment of anaphylaxis. Adult intramuscular dose is 0.3 to 0.5 ml of 1:1,000 concentration. This should be given in the lateral aspect of the thigh by intramuscular injection. The dose can be repeated every 5 to 15 minutes, depending upon the response, for 3-4 doses. The same is true for children except the dose is 0.01 mg per kg (AAAAI Ask the Expert, 2012).
What are the 4 standardized allergen extracts?
(A) Dog
(B) Trees
(C) Cat
(D) Molds
(E) Dust Mite
(F) Grass
(G) Ragweed
The 4 standardized extracts are Cat, Dust Mite, Grass and Ragweed.
References
Allergen immunotherapy safety: Characterizing systemic reactions and identifying risk factors. Rank, Mathew A.; Oslie, Corrine L.; Krogman, Jennifer L.; Park, Miguel A.; Li, James T. Allergy and Asthma Proceedings, Volume 29, Number 4, 7/8 2008 , pp. 400-405(6).
Evaluation of near-fatal reactions to allergen immunotherapy injections. Amin HS, Liss GM, Bernstein DI. J Allergy Clin Immunol. 2006 Jan;117(1):169-75.
Anaphylactic reactions during immunotherapy. Rezvani M, Bernstein DI. Immunol Allergy Clin North Am. 2007 May;27(2):295-307, viii.
Allergen immunotherapy: A practice parameter second update. JACI, 2007 (PDF).
Anaphylaxis: A Short Review
Rate of systemic reactions during subcutaneous immunotherapy: 0.28% per injection
Mnemonics: Anaphylaxis
Mind Maps: Anaphylaxis
Anaphylaxis guidelines by World Allergy Organization. JACI, 2011.
Published: 02/12/2009
Updated: 06/12/2012
Published: 02/12/2009
Updated: 06/12/2012
Subscribe to:
Posts (Atom)